
Track and Field Energy Systems
ATP is the key for energy production in athletics. Bioenergetics describes the flow and transformation of carbohydrates, fats and proteins into energy that can be used to perform work. The potential chemical energy from the metabolism of food sources are stored in the bonds of various molecules that can be converted into kinetic mechanical energy.
To generate kinetic mechanical energy, work must be performed. The release of energy from the chemical reactions generate muscle contractions (work). During work, the molecules of the chemical bonds are broken apart and transformed. With movement, skeletal muscle transforms chemical energy into mechanical work.
Energy Production
Chemical energy from food sources must be transferred into adenosine triphosphate (ATP) to become useable energy within the body. The ATP molecule consist of one large complex molecule called adenosine that is linked to three phosphate groups. ATP is a biochemical molecule to store and produce energy. ATP is stored in the muscle cells for immediate use, ATP can also be generated for energy by the phosphagen system, glycolysis and aerobic respiration. Metabolism to generate ATP from the different energy systems is based on the intensity and duration of exercise.
Muscle Contractions
Muscles contract because of stored energy in the form of ATP. ATP is formed in the muscle by bonding inorganic phosphate (Pi) to ADP. The bond is broken by activation, potential energy is released causing the muscle to contract.
ATP Production and the Phosphagen System
Immediate energy is primary produced by resting ATP, creatine phosphate and the enzymes creatine kinase and myokinase (Stone, Stone, Sands, 2007). ATP stored in the muscle is readily available to start movement, regardless of the intensity.
Only a small amount of ATP is stored for a few seconds of energy. Work from the energy supplied ATP within the muscle cells will be depleted within a few seconds during high intensity exercise. ATP must be regenerated by ADP and creatine phosphate to maintain muscle activity by a process called phosphocreatine hydrolysis to maintain maximal activity.
Read more on the Phoshagen Energy System from Science Direct
ATP hydrolysis is a catabolic reaction process that produces work from stored energy: ATP + water = hydrolysis
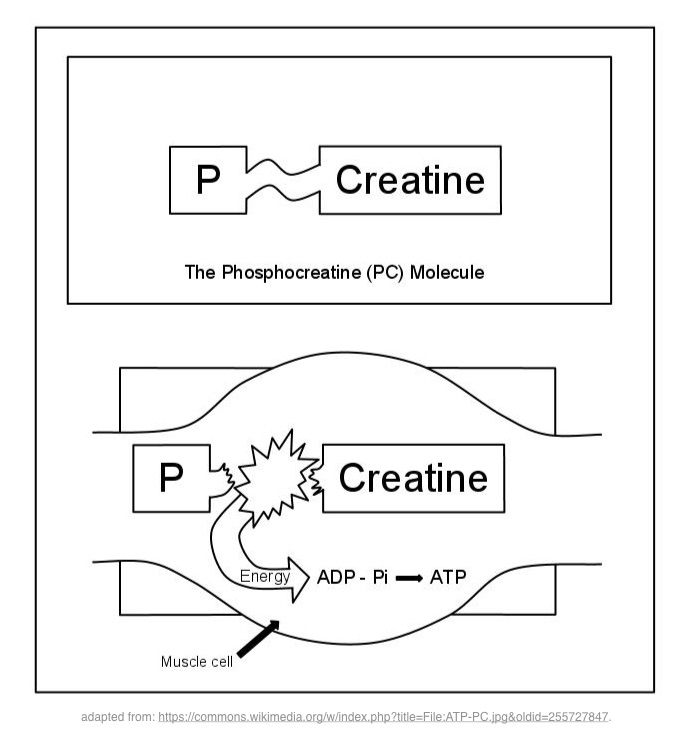
Creatine phosphate is an energy reservoir inside the muscle cell that will enable the regeneration of ATP. The enzyme creatine kinase catalyzes the conversion of ADP to ATP, phosphate is transferred from creatine phosphate to ADP to reform ATP.
Myokinase, the third energy source in the ATP/phosphagen system interconverts ADP to ATP to maintain high intensity exercise. Myokinase shuttles ATP to be used for muscle contractions and diffuses the generated adenosine monophosphate (AMP); two ADP molecules join to form ATP and AMP.
| Phosphagen System ATP Supply | Reaction |
| Resting ATP + H2O | Myosin ATPase |
| Creatine Phosphate + ADP | Creatine kinase |
| 2 ADP | Myokinase |
To continue high intensity exercise, additional ATP is required from the chemical breakdown of fuel sources, known as substrates. Glucose and glycogen are the primary substrates to be catabolized as an energy source to maintain movement.
Speed training will improve the effectiveness of the phosphates energy system
ATP Production and Glycolysis
Glucose is a small molecule in the form of sugar from carbohydrates that can be used for immediate energy or can be stored in the muscles and liver. When multiple glucose molecules are stored together, the more complex molecule glycogen is formed.
When the body needs energy for work, glucose can be used immediately, or glucose stored as glycogen can be broken apart and released into the bloodstream to be used as fuel. The additional step to breakdown glycogen to glucose is called glycogenolysis.
Glucose is transformed to produce energy by glycolysis.
After the process of glycolysis is complete, one molecule of glucose is broken down into two molecules of pyruvate that will converted into an additional energy source. The output of pyruvate will be converted to lactic acid or acetyl-CoA, the conversion is based on the level of oxygen available.
Without sufficient oxygen, pyruvate is broken down to lactic acid. The anaerobic degradation of glycogen causes lactate to increase within muscle and slowly diffuses into the blood stream. The pH levels within the muscle fibers decline because of the increase in lactate and hydrogen ions in the blood (Reilly, 2007). When lactate uptake exceeds lactate removal, the quality of muscle activity decreases.
When ATP is split for energy, a hydrogen ion is released, the accumulation of hydrogen lowers the blood pH creating an acidic environment in the muscle cells. To restore pH balance in the muscle cells, the unwanted byproducts must be reduced or eliminated. A change in exercise protocol is required (lower intensity movement or rest) when the muscles get irritated in the acidic environment caused by the influx of hydrogen ions.
Anaerobic Glycolysis
Anaerobic glycolysis is replaced by aerobic glycolysis as a more dominant energy source in exercise after 90 seconds for longer duration exercise and less intense exercise (Verkhoshansky & Siff, 2009).
In anaerobic glycolysis, lactate is the normal endpoint, however minimal amounts of lactate can be removed by oxidation or cleared and transformed back into glucose by glycogenolysis or transported to the blood (Juel, et el, 2004). Lactate can exit the muscle cells into the liver to be oxidized back to glucose via the Cori cycle (Gunnerson, Harvey, Pinsky & Talavera, 2018).
Note: The activation of glycolysis results in the production of lactic acid. Lactate does not accumulate during lower intensity exercise with a readily available supply of oxygen.
Coaching Point: all field events and some short sprint races in track and field are 100% anaerobic, dependent on the high energy phosphagen system or a combination of the high energy phosphagen energy system and the glycolytic energy system.
Aerobic Glycolysis
In an oxygen rich environment, pyruvate is converted to acetyl-CoA by a process called pyruvate decarboxylation. The breakdown of glucose into acetyl-CoA is part of the metabolic process dependent on the continuous use of oxygen. Acetyl-CoA is the main reactant to engage the TCA cycle and oxidative phosphorylation.
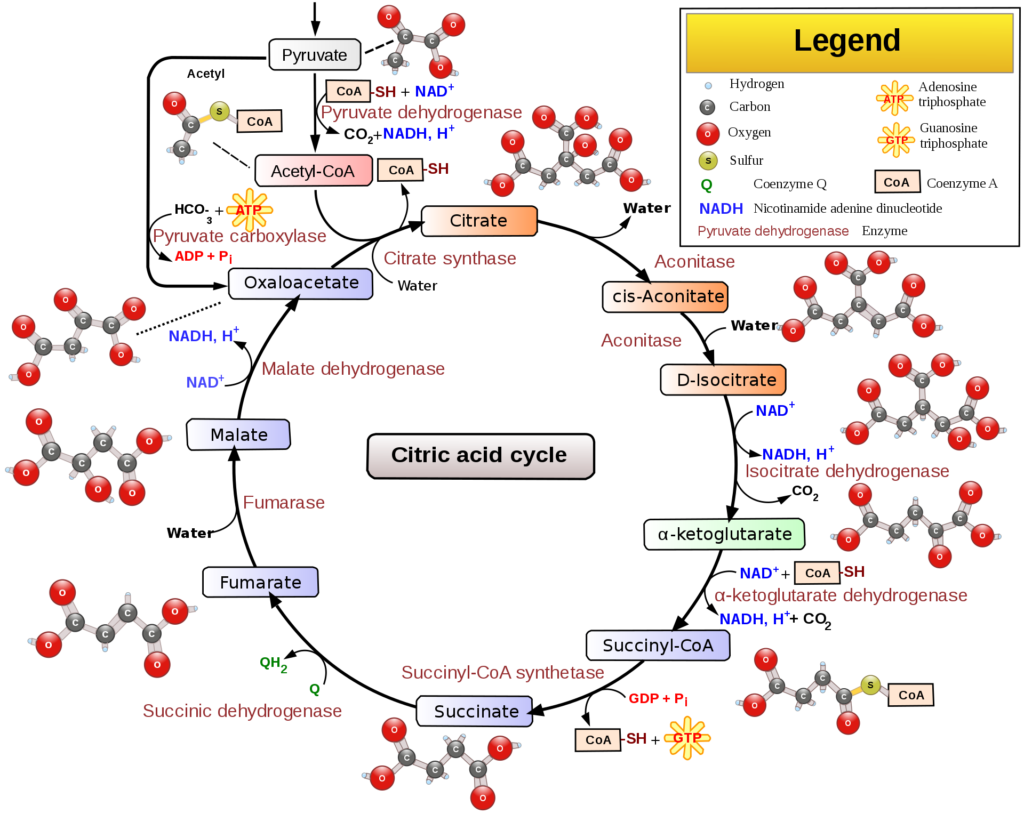
Tricarboxylic Acid Cycle (TCA Cycle)
After glycolysis, the two molecules pyruvic acid are altered to form two molecules of acetyl-CoA for the TCA cycle.
Three molecules of NADH, one molecule of FADH2 and one molecule of ATP are generated, while three molecules of carbon dioxide are released as waste products during one turn of the TCA cycle. One glucose molecule will provide two turns of the TCA cycle since two acetyl-CoA molecules are formed for each glucose molecule consumed.
NADH and FADH2 will move onto the next step of aerobic respiration, oxidative phosphorylation (electron transport chain and chemiosmosis).
Note: the tricarboxylic acid cycle (TCA cycle) is also called the Krebs’s cycle after Hans Krebs, one of two researchers that discovered the process. The TCA cycle is also referred to as the citric acid cycle because the metabolic pathway is derived from citric acid, a type of tricarboxylic acid called citrate.
Oxidative Phosphorylation
Electric Transport Chain and Chemiosmosis
The electron transport chain receives electrons from NADH and FADH2 that are passed through a series of electron transporters that undergo redox reactions between protein complexes that regenerate NADH to NAD+ and FADH2 to FAD. Oxygen is attached to the energy reduced electrons which reacts to two protons (hydrogen) to form H2O.
Protons are pumped through specialized channels that travel in the mitochondria to diffuse protons down an electrochemical gradient through the enzyme ATP synthase. The process of energy production via the proton gradient is known as chemiosmosis. ATP is generated by the movement of hydrogen that catalyze the pairing of a phosphate with ADP to form ATP.
Energy Production
Anywhere from 30 to 38 molecules of ATP can be generated from the oxidation of one glucose molecule. 38 ATP from one glucose molecule is highest theorical yield. The lower estimates of ATP assume the energy cost of transporting pyruvate, phosphate and ADP into the mitochondria that require stored energy for movement.
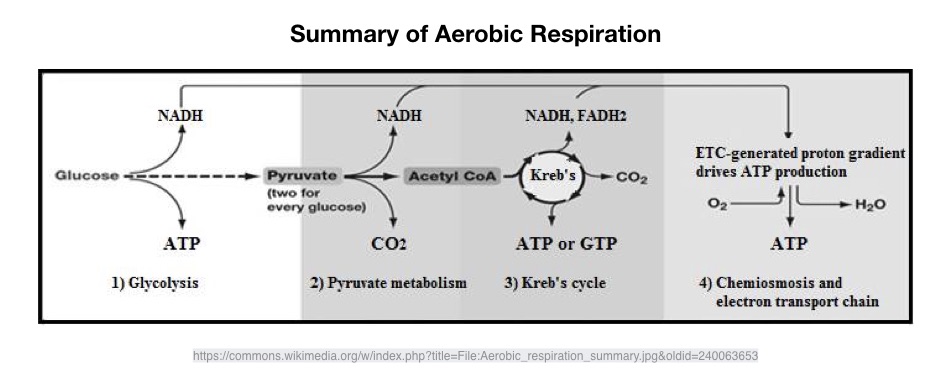
The production of energy is interconnected, each system operates on a continuum. Each energy system has different qualities based on power and capacity, the anaerobic-aerobic energy split is determined primarily by the availability of oxygen as a result of the intensity and duration of exercise.
Energy System Review
The high energy phosphagen energy system and the glycolytic energy system are used to preserve ATP when ATP is used rapidly. The synthesis of ATP without the presence of oxygen are anaerobic pathways. Anaerobic pathways occur outside the mitochondria in the cytosol of the muscle fiber.
When ATP resynthesizes at a low rate, the aerobic metabolic pathway can maintain a constant ATP level via oxidation (oxidative phosphorylation). The synthesis of ATP from the oxidation of glucose/glycogen or other fuels is an aerobic pathway. Aerobic pathways occur inside mitochondria in each muscle fiber.
Here is one of our favorite articles on energy systems from PT Direct








